pin2
Home » pin2
Oral Peptide Delivery Challenge
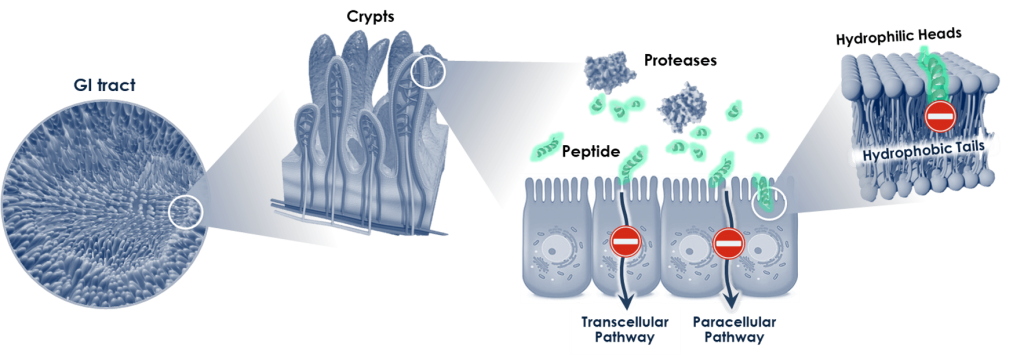
Currently, most protein therapies are administered via frequent intravenous, subcutaneous or intramuscular injections. In chronic diseases where patients require persistent management, these cumbersome, often painful and high-priced injections can create a major treatment gap.
From a technical standpoint, oral delivery of therapeutic proteins is challenging due to the enzymatic degradation within the gastrointestinal tract and poor absorption into the blood stream due to the proteins’ polarity and molecular weight.
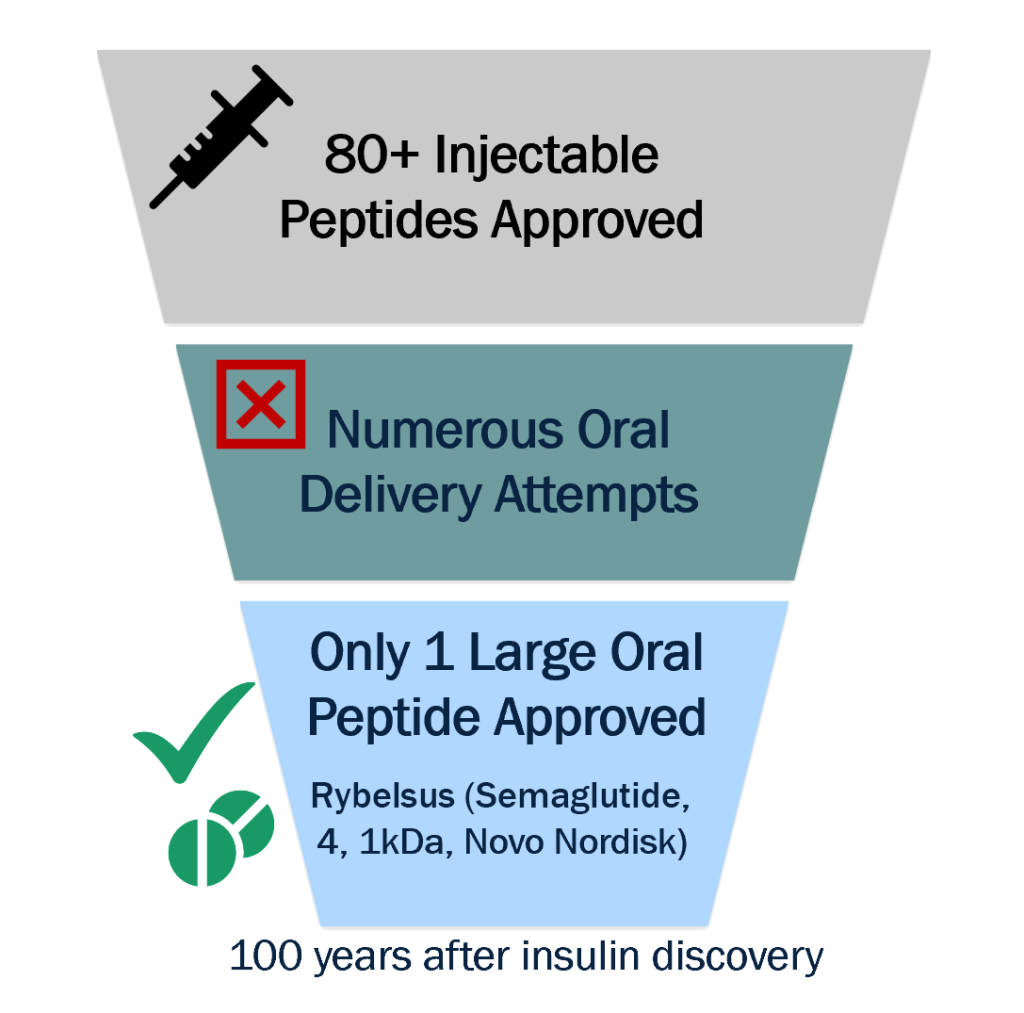
Oral Delivery of Peptide Drugs Has Lagged
Out of >80 approved injectable peptide therapies, there is only one approved oral peptide >4kDa (GLP-1, Rybelsus®)

Oral Delivery of Peptide Drugs Has Lagged
Out of >80 approved injectable peptide therapies, there is only one approved oral peptide >4kDa (GLP-1, Rybelsus®)
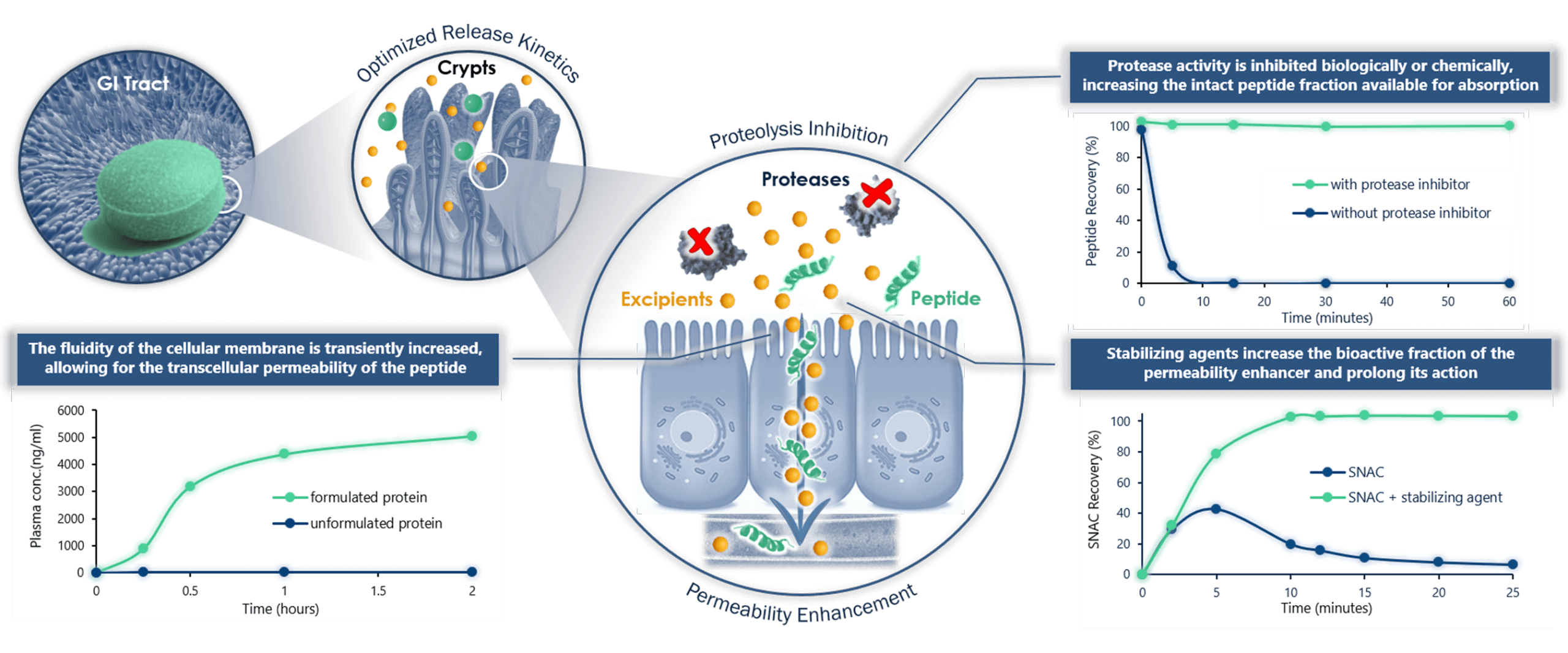
Our Solution
N-Tab™ Entera’s Proprietary Platform is designed to simultaneously stabilize the peptide in the gastrointestinal tract and promote its absorption into the bloodstream
Our Solution
N-Tab™ Entera’s Proprietary Platform is designed to simultaneously stabilize the peptide in the gastrointestinal tract and promote its absorption into the bloodstream
Unlocking Value with the N-Tab™ Platform:
Preferred by Patients
Oral meds are easier to take and more convenient in chronic conditions
Daily Tablet Unlocks Access to New Drug Classes
Tablet format makes new therapies possible
Platform Designed to Address This Unmet Need
Built to overcome the key barriers to oral biologics - stability, absorption, and bioavailability
Unlocking Value with the N-Tab™ Platform:

EB613
PTH 1-34
EB613 (Oral PTH(1-34),teriparatide) is being developed as the first oral, osteoanabolic (bone building) once-daily tablet treatment for post-menopausal women with low bone mineral density (“BMD”) and high-risk osteoporosis. EB613 is intended to provide an oral anabolic treatment earlier in an osteoporosis patient’s journey to increase skeletal mass, reduce the risk of fracture, potentially limit the progression of the disease, and its associated disability and mortality.
Read MoreEB612
PTH 1-34
The EB612 program is being developed as the first oral PTH(1-34) tablet peptide replacement therapy for hypoparathyroidism. We are currently testing new generations of our N-Tab™ Technology with the naked PTH(1-34) peptide to assess the effectiveness of once or twice a day dosing regimens, as well as collaborating with a third party on another peptide in this field.
Read MoreOXM
GLP-1 & Glucagon Agonist

First GLP-1/Glucagon Agonist (Oxyntomodulin) Peptide Tablet Candidate for Obesity and Metabolic Diseases
• The program is focused on developing the first oral dual agonist GLP-1/glucagon peptide as a potential once-daily treatment for patients with obesity and metabolic disorders combining OPKO’s proprietary long-acting oxyntomodulin analog (OPK-88006) and Entera’s proprietary N-Tab™ platform. In September 2024, Entera and OPKO Health jointly announced topline pharmacokinetic/ pharmacodynamic (PK/PD) results for the oral oxyntomodulin (OXM) tablet program.
• Oral OXM exhibited significant systemic exposure across two in vivo models, a favorable PK profile and bioavailability. The high plasma concentrations with prolonged systemic exposure were consistent with the reported half-life for semaglutide (Rybelsus®), the only approved oral GLP-1 analog. Oral OXM showed a statistically significant reduction in plasma glucose levels compared with placebo.
• In March 2025, we entered into a collaboration and license agreement with OPKO relating to the preclinical and clinical development of the Oral OXM program. The companies expect to file an IND with FDA late in 2025/ early 2026.
GLP-2
Long Acting GLP-2

First GLP-2 Peptide Tablets for Short Bowel Syndrome
Given the challenging compliance rates attributed to injectable GLP-2 therapy and heterogeneity of short bowel syndrome (SBS) patients, we believe a daily tablet format may address a significant unmet need in treating and titrating SBS patients more effectively than injectable alternatives.
In September 2023, we entered into a research collaboration with OPKO Biologics, whereby OPKO supplies its proprietary long-acting GLP-2 peptide for short bowel syndrome (SBS) using our proprietary N-Tab™ technology.
EB613
PTH 1-34